OT: Covid-19 Neck Sharpies Part II: The Tests, The Tests, The Tests
Previously: Part I Hello: Antibodies (April 25)
You're back for more. The first part didn't convince you you made a mistake not going into science, but this one gets bloody, so you may reconsider. Same apologies as last time. Not posting as a Diary (yet) because that's for teenage girls but anyway DO NOT TRUST REGINA GEORGE SHE IS A FUGLY SL... (hey, we went over this last time, stop using that word).
___________________
PART II. HOW DO THE VARIOUS COVID-19 TESTS WORK, AND WHAT DO THEY TELL YOU?
TL;DR: Go see the charts at the end, Mr. Rush-Rush. Also, buy some corn holders.
The problem with how testing is being widely reported is that they're lumping things together that are really distinct tests answering distinct questions, or the same question in different ways. This is bad and confusing to people who consume news-like substances.
There are roughly 4 kinds of tests out there right now. You are basically asking one of two questions with each test:
Question the first: Am I infected right now?
Question the second: Was I exposed/infected in the past?
TEST #1. RT-PCR ("Reverse Transcription Polymerase Chain Reaction")
Answers the Q: Am I infected right now?
By looking for: Viral RNA
PRO: Technology has been around since the 80s, most larger hospitals can run it
CON: Slow-ish (roughly 8 hours), hardware is too pricey for home use, CDC was taking 5 days to flip results for most of the crisis because kit reagents were unavailable, false negative rate is a problem
You May Remember Me From Such TV Shows As: CSI [Insert Your Murderous City Here], Forensic Files, and NCIS:[Insert Your Murderous City Here if CSI Skipped You].
PCR is the basis of modern forensic investigation. This is because you can take a tiny amount of DNA you find on a cigarette butt and use a pricey benchtop machine called a thermocycler to amplify it with a chain reaction process until it turns into a big pile of IT WAS THAT GUY.
How it works: The "RT" part of the equation flips the usual flow of biology in reverse: instead of DNA to RNA like every living thing (except retroviruses, which are not quite a living thing but ya feel me, dog?), you start with the Coronavirus RNA and throw it in reverse to make DNA, then turn on the molecular Xerox copier for a few hours (the "PCR" part).
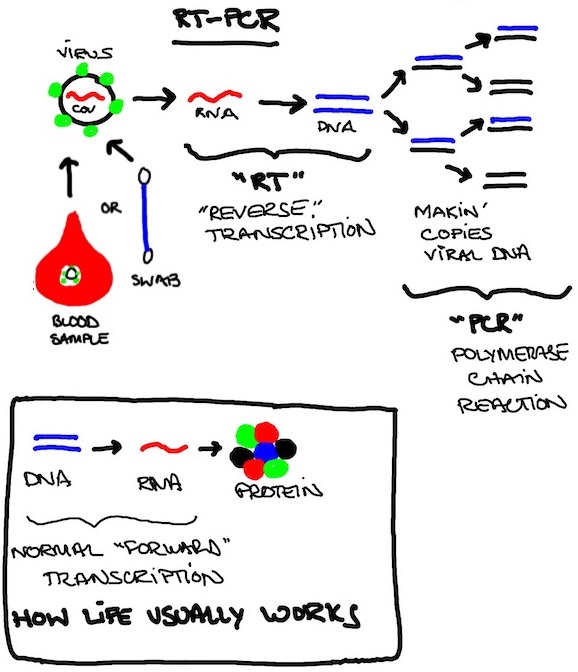
Q. Why even bother with the "RT" part? It just embiggens the acronym.
It's because DNA is stable and much easier to work with, store, amplify, and clone dinosaurs from. RNA is fragile and temperamental. Here today, gone later today. To Arizona St. or the G-League, probably. Too soon?
Worth knowing: RNA's instability means a Coronavirus isn't going to hide out somewhere for months or years or linger after the acute infection is over, like DNA-based viruses e.g. HIV or HSV. If you're RT-PCR positive, there is active virus cranking somewhere in you. Coronaviruses have no "off switch" and this is no long con like The Sting. The standard Coronavirus plan is WE ALL RUSH THE KWIK-E-MART AND GRAB THE BEER AND BREAK A BUNCH OF SHIT AND RUN LIKE HELL CAUSE I THINK I HEAR SIRENS ALREADY.
Digression: PCR was a pretty ingenious setup. The creator, Kary Mullis, won a Nobel Prize for it in 1993. He assigned the patent rights to his employer Cetus, who, ahem, generously gave him a $10,000 bonus, then they turned around and sold those rights for $300,000,000. This message brought to you by CALL ME I'M A PATENT LAWYER.
TEST #2. Abbott "ID Now" Platform
Answers the Q: Am I infected right now?
By looking for: Viral RNA
PRO: Works super fast compared to RT-PCR, supposedly 5-15 minutes instead of many hours (fn6)
CON: Not fully vetted in the field (available under FDA's Emergency Use Authorization, which skips a lot of testing), platform hardware not common in labs like PCR, does one cartridge at a time so throughput doesn't scale easily, initial independent study of accuracy had high false negatives (fn7)
You May Remember Me From Such TV Shows As: COVID Task Force Daily Presser & Shitshow
The platform tech has been around for a minute but the COVID-19 application is new. Abbott is pumping it up and got the aforementioned plug from the White House briefing, but it's not available widely. Still, if they get the kinks worked out, would be a much faster alternative to RT-PCR tests because it doesn't reverse RNA into DNA and never cycles through different temperatures.
Allegedly. Good time to apply marketing hype discount.
How it works: The test kit uses short primers to find the virus RNA and make some quick copies of a sequence from a single gene (fn8). Those copies are lit up with a fluorescent probe and detected by the toaster-sized machine in a fraction of the time it takes for PCR to pile up a detectable amount of DNA.
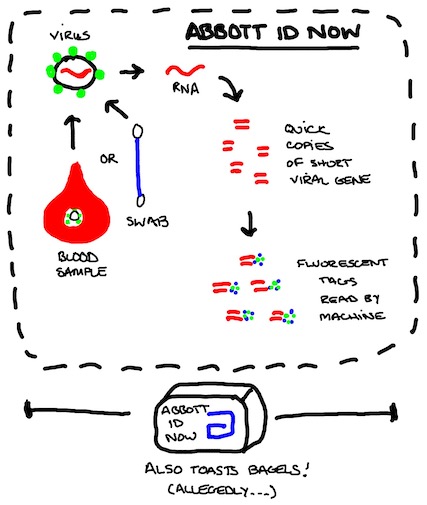
As above with RT-PCR, recall Coronaviruses are RNA viruses, so they're here for a good time, not a long time. If your infection is clear, there are no residual leftovers that show up positive in the Abbott test either.
TEST #3: Antibody Test Type 1 (anti-virus Antibody in Test Kit Seeks Patient's Virus)
Answers the Q: Am I infected now?
By looking for: Intact virions or envelope proteins
PRO: Suitable for a home-based test, well-vetted technology
CON: Not quite as sensitive as PCR assay (some kit manufacturers will disagree) especially early on in infection
Let's make like Katz's Deli and make us some sandwiches. This is a classic assay that you can do in a couple of different ways, but the main principle is:
STEP ONE: immobilize the thing you're looking for with a highly specific (very picky) and high affinity (very sticky) antibody for that thing
STEP TWO: Attach something else to that complex with a signal so you can read it out (a "secondary antibody" that you already know will bind the target and comes with a label of some kind (red dot in the illustration below)
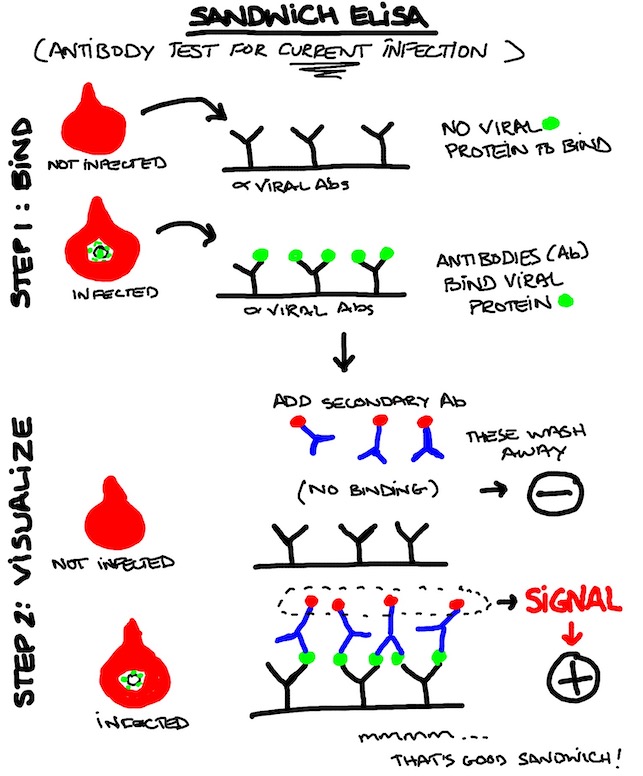
Pretty useful test, and you conceivably do it at home. You might be able to do it without a blood sample at home using a swab, but your swab collecting skills need to be really good and it might be a less user-friendly test for millions of people to perform at home. People who can't put links in MGoBlog posts might not be trusted to perform the protocols 100%. I'm just sayin'.
Take a breath.
Now for the big one.
Now, we get to the thing that is probably going to decide when we get back to "normal" as that term is going to be understood for a while.
So we beat on, boats against the current, borne back ceaselessly into the past, where we may or may not have once been infected by SARS-Cov-2.
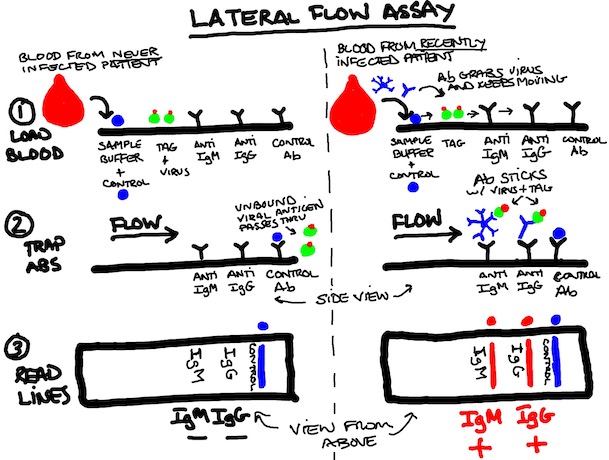
TEST #4: Antibody Test Type 2 (Viral Protein in Test Kit Seeks Patient's anti-virus Antibody)
Answers the Question: Was I exposed/infected previously?
By looking for: Antibodies against the virus
PRO: Simple test, can be run at home or in big labs with large throughput of samples
CON: Interpreting the results and drawing conclusions can be... fraught.
I predict almost everyone on this board will be ordering this kit from Amazon to perform at home by the end of the year (or a lot sooner), so you may as well learn how this sucker works.
Basically, the principles here are the same as the ELISA above but you're playing with what stays fixed, and what moves and when. This setup isn't much different than a home pregnancy kit with a couple drops of blood used instead of urine. PRO TIP: You will not get reliable results on your COVID-19 test if you just remove it from the box and take a piss on it.
1. STEP ONE: LOAD BLOOD SAMPLE. Fingerstick and trickle a little blood into the collection area at the bottom of the strip. Add a little buffer and let everything wick through the strip (left to right below). At this point, you have your blood's antibodies (which may or may not be specific for SARS-CoV-2) moving along with a control protein (blue dot) and purified viral protein (green) tagged with a dye (red dot). No, that's not an olive, please stop asking. While you're waiting for the test to work, you may want to take advantage of the blood dripping from your finger.

2. STEP TWO: TRAP SPECIFIC ANTIBODIES. anti-SARS-Cov-2 Antibodies (IgM, IgG) will bind to the (green) viral protein and move along with them until they hit the anti-IgM antibody area or the anti-IgG antibody area (yes, antibodies against antibodies are totally a thing). Non-specific (non-picky) antibodies for other things we don't care about will just flow off the end, as will unbound viral proteins if there are no specific antibodies to grab them.
3. STEP THREE: READ THE LINES, NOT BETWEEN THEM. First, you make sure your control line is there, or you throw the strip out. The positive control should work every time because it is independent of the specific antibodies you're looking for, and because the definition of a positive control is that it works every time. THIS IS NOT SEX PANTHER COLOGNE. "60% OF THE TIME IT WORKS EVERY TIME" IS NOT SUFFICIENTLY RIGOROUS FOR VALIDATED TESTING. The dye stuck to the viral protein will aggregate at the lines if specific (anti-SARS-Cov-2) antibody for that protein is present in the blood sample.
NON-INFECTED PERSON (left) INFECTED PERSON (right)

Nice computer animation of the above with a surprisingly excellent electronic soundtrack (they're not searching for antibodies in the sample but principles are entirely the same)(run time 1:53).
Here's a link to a video showing how a test like this would go down for the patient (in this case, it looks like the patient tests negative for both anti-SARS-Cov-2 IgM and IgG)(run time 1:18).
So you end up with a variety of potential results. And things look simple enough... until you try to go from "what is the result?" to "what does this mean in the big picture?"
THE RESULTS, THE RESULTS, THE RESULTS

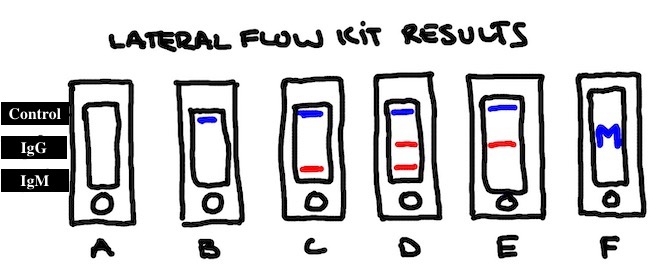
A: No Lines. Your control didn't work, so this one is meaningless.
B: Control only. You have tested negative.
C: Control + IgM. You resolved a past infection quickly (Paul Blart and weird guys to the rescue) or you're in the middle of one right now. You'll probably want to take another test in a week or so.
D: Control + IgM + IgG. You had a full blown infection, and probably recently. Or you have one now. How are you feeling?
E: Control + IgG. If you're feeling well when you took the test, you've probably fended the sucker off. The best case scenario (which we hope but don't know) is that you're not probably not going to get sick again unless a different strain starts a comeback world tour.
F: Block M: Time to source your kit from someone other than Wolverine Devotee.
Pulling everything together at this point seems like a good idea. Yes. Let's.
Chart? Chart. Charts. Two for the price of one. (I’m going to go ahead wager this will not translate well to portrait mode on a phone. Damn the torpedoes)
Chart the First: The Tests (some assembly required)
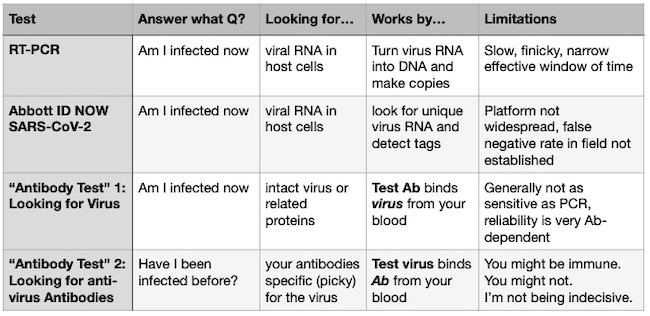
With PCR and ID NOW, recall that you’re probably not going to pick up the virus at the beginning and ends of the infection. With Antibody Test 1 (Looking for Virus), at the end you might be picking up a little more because there is some leftover protein debris from the virus that hasn’t been cleared even though there are no more infectious particles replicating. Remember, RNA disappears fast. Proteins might hang out a little longer.
Also helps to bear in mind this visual reminder from Covid-19 Neck Sharpies Part I of how we're expecting antibodies to come and go. Since we're assuming this is the first time we've seen the virus, the left side of the below graph is the relevant one here:
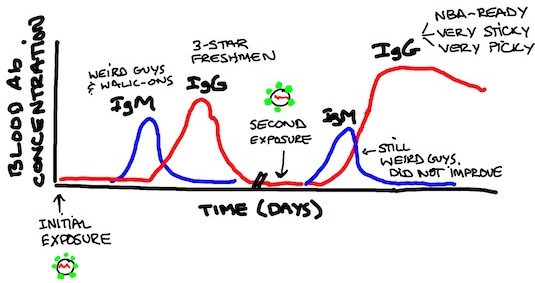
Chart the Second: The Results (some SWAG* required).
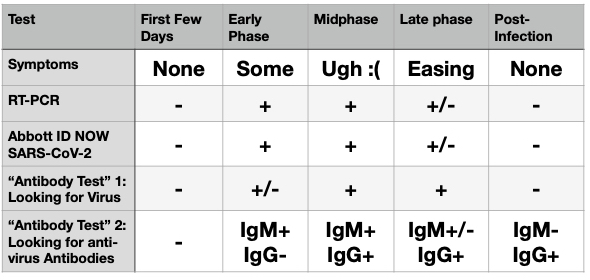
*scientific wild-assed guessing
Q. So, assuming everything worked exactly as we expected (fn9), and I have lots of antibodies, I can pack into The Big House in the fall with 110,000 people who have also tested antibody-positive, and no worries about anything but our untested offensive line and Y NO DEFENSIVE TACKLES?
Yeah, so about that... would you settle for maybe?
NEXT UP IN COVID-19 NECK SHARPIES : PART III. "IMMUNE." YOU KEEP USING THAT WORD.
Earlier I said expect this Monday. By which I meant, don't expect it Monday.
__________
Footnotes
8. See Abbott Labs Covid-19 ID NOW Product Insert (at page 2) LINK TO PDF
9. Yeah, everything working as expected is... maybe optimistic? Coronavirus Antibody Tests: Can You Trust The Results? (New York Time, April 24, 2020)
For the past few weeks, more than 50 scientists have been working diligently to do something that the Food and Drug Administration mostly has not: Verifying that 14 coronavirus antibody tests now on the market actually deliver accurate results.
These tests are crucial to reopening the economy, but public health experts have raised urgent concerns about their quality. The new research, completed just days ago and posted online Friday, confirmed some of those fears: Of the 14 tests, only three delivered consistently reliable results. Even the best had some flaws.
What are your thoughts on this potential treatment:
Heard of it but haven't read any primary papers about it. There is really no shortage of candidates based on reasonable proposed mechanisms of action right now, but there's just a lack of actionable data. My hope is to pull a list of the candidates together in a couple of weeks and see where they stand. The literature has been flying fast and furious-- very challenging trying to keep up.
It was my understanding there would be no science.
I thought the rules here were no politics, religion or science. MODS...delete all this. This board is about bemoaning football recruiting. Can't get enough of that.
/s
i remember my first PCR case, a rape case, way back when that was novel technology. you, dr. sopwith, were like what, 5 then? how many is that in dog years?
again, great stuff and you are a champ to take the time to make such a detailed, readable, and light-hearted write up. amazing how much of the mgoblog type of humor can make microbiology fun.
I remember the first time I ever heard of PCR or DNA fingerprinting was this true-crime book by Joeseph Wambaugh called "The Blooding." If memory serves this small English town in the 1980s ended up taking blood samples from most of their male population and were able to solve a murder for the first time anywhere using PCR. It must have all seemed like hocus-pocus to both criminals and juries back in the day.
April 27th, 2020 at 10:01 AM ^
I was in high school AP Biology in 2007-08, and we were doing experiments using PCR. My high school teacher commented that he first saw PCR when he was in college in the early 90s. Science and technology move fast.
Aside, my HS teacher's college professor started teaching science before DNA was discovered!
TLDR
good stuff. I will have to read that in greater detail again when I have more time.
Do you know who is commercially working on #4?
The answer may be "too many people." There may be a lot of junk tests in the system for a while. Check that last footnote and click on the embedded link in that quoted section of the NYT article. I believe that has the names of the 14 companies' kits that were quality tested.
Honestly, the best piece I’ve read on the subject, and I’ve probably read as much as it’s possible for a non-medical professional to.
Awesome information. It probably should be noted that the primary reason for false negative rates in the PCR tests are because of sample collection and not the actual test process itself. I think you would agree that if you give, say a Roche PCR system, a good sample (meaning the swab was inserted and made contact with a virus carrying surface) it will provide a highly accurate result. If the collector of said sample doesn't contact the proper surface (and said surface is way up in the ole snooter) then a false negative can absolutely be spit out.
Also, while PCR tests do take a while to run, they are also scalable to run pretty high volumes at a time so still the best we have to test the masses on the do I have it or not side.
That's a great point. PCR is superbly accurate if all the reagents and samples are properly prepped. But I'm getting more and more skeptical of the sample collection side of things.
The Cepheid RT-PCR based Covid tests run in <1 hour and seem reliable. They can't make enough to test the entire country, but are important for situations where time really matters (ED triage to inpatient units).
There are some in-house RT-PCR tests at various research-institution linked hospitals that seem relatively fast, but that would be even faster than those. Good option to have, I know about their GeneExpert platform but hadn't seen much on the Covid-19 side.
Great job. This explains things nicely and in an easy to understand manner. The chart demonstrates the big problem with testing, even with very accurate tests. NO tests can detect the virus in the first few days, yet those people can spread the virus. Testing (and screening) can still miss the first few days of a worker or a customer with the disease who can then spread it throughout business, restaurant, office, etc. Even with readily-available accurate tests, a highly infectious disease like this can spread through a population quickly. It is a difficult problem without a good solution at this time.
NO tests can detect the virus in the first few days, yet those people can spread the virus.
I’m curious ... if NO test can detect the virus in the first few days, how can one determine if those people can spread the virus? Am I missing something?
Also, why only mention “false negatives” with regard to the PCR tests? As I understand it, there are false positives as well that can arise as cycles are repeated until a “positive” result is found. Every cycle increases the amplification, and thus the “noise” presented by the result. Is there a baseline number of cycles that is a bright line between positive and negative?
In some cases you can establish the chain of infection with contact tracing, which might tell you "sick individual X had close contact with Y on Monday, on Tuesday Y passed it to Z while Y was still asymptomatic."
In general you don't expect to pick up the positive people in those first few days but you could either get lucky with your sample collection or just test someone who had exposure to a particularly heavy viral challenge on day 0. So it's not impossible, but it's unlikely with the current tests.
So really a non-answer then, huh?
Shouldn’t the number of cycles required to establish a “positive” test result be included with the result?
It’s a great question, I wonder the same thing. You would expect that a lower viral load would lead to fewer infections, but who knows. It seems like there are a few super spreaders (the Biogen exec, patient 31 in South Korea) that are extreme outliers, would be nice to know how that correlates with viral load.
April 26th, 2020 at 10:33 PM ^
You're not running quantitative PCR here, just getting an up or down. If there's any viral RNA, that's a positive test. The number of cycles you took to get there doesn't have a bearing at that point.
April 27th, 2020 at 12:36 AM ^
Unless there's a chance that replication error can lead to a false positive. That seems unlikely unless the test identifies RNA sequences that are "close" to something innocuous, where "close" is defined in terms of a small number of nucleotides' difference. I'm not an expert in polymerase error rates, but what I'm seeing in a quick study suggests that this shouldn't be a major source of concern; error rates seem to be pretty low, and errors of more than one nucleotide appear to be vanishingly small. So, yes, I don't think the number of cycles makes a difference.
Sopwith, this one (the Raquel Welch reference notwithstanding) might be better than the last. I am looking forward to your magnum opus.
Probably going to end up being the shortest one now that I've gotten all this stuff out of the way. ;) Anyway I'm taking a break and catching up on non-Covid related TV.
Let me know how that works out for you. :-)
BTW, my physician has me on a course of Ta1 to moderate inflammatory response and prime the T-cell pump in the event I get infected. Thoughts? A couple of friends who are physicians have mixed feelings on the directive.
April 26th, 2020 at 10:29 PM ^
I'm not a clinician so that's outside my intended commentary, but I can see the rationale.
I have no intention to buy a home test kit on Amazon :), but I agree with the rest of what you wrote. There's one additional testing topic that I think is important for the "test everybody" crowd to understand, and that's Bayesian probability theory, and particularly the concepts of sensitivity and specificity. I wrote a post on this recently, but I'm sure it's lost somewhere in the long discussions since.
Specificity refers to the true negative rate; highly specific tests have few false positives. Sensitivity refers to the true positive rate; a highly sensitive test has few false negatives. For an antibody test -- the one that you'd expect people to be buying -- false negatives are less important than false positives; you're taking this test because you want a positive result, and you need to be confident in that result, because you're presumably making behavioral choices based upon it (this goes into the concept of immunity that you've got listed for Part 3).
The key point to remember is that if a test is 95% specific, and you get a positive result, it does not follow that you are 95% likely to have antibodies. In order to evaluate a positive test result, you need to know not only the specificity of the test, but also the rate of true positives in the population.
As an extreme example, consider a test that is 95% specific and 99% sensitive -- a very good test. Let's take a rare syndrome with a one in 10,000 occurrence rate. If you have a million people, you expect to have 100 people in the population with that syndrome. If you run a million tests, you're going to get 99 true positive tests (one false negative, based upon the 99% sensitivity). You're also going to get 49,995 false positive tests, for a total of 50,094. If you get a positive test back, and the doctor tells you, "I'm sorry, but this test is 95% accurate, so you must have the syndrome" -- it's just not true. In fact, the chance is 99 / 50094, or 0.2%, that you have the syndrome -- the false positives overwhelm the real ones.
On the other hand, suppose we're talking about a COVID-19 antibody test once they are 50% prevalent in the population, and keep the same test accuracy. Now, out of those same million people, you'd expect 500,000 to have the antibodies. Of those, you'd get 495,000 true positive tests (5,000 false negatives), and you'd also get 25,000 false positive tests from people without antibodies. But now, if you got a positive test, the chance that it's real has skyrocketed -- 495,000 / 520,000, or 95.2%.
TL/DR: Before you buy a test, you need to know how prevalent the antibodies are. Unless we find tests that are amazingly specific (99.9%+) -- which is pretty unlikely -- don't bother buying a set unless the prevalence is at least 20%. Otherwise, you're just wasting your money.
Note: I edited this post for clarity; MGoProstate is correct that I was using the terminology incorrectly. The calculation is unchanged, however.
The spirit of the argument is correct, but I fear you may have your PPV, NPV, sensitivity, and specificity confused. The bottom line is that few tests are perfect, and the imperfection of the test should match the intended use, i.e. a population screening test should not have a high false positive rate if the prevalence of the disease is low.
a population screening test should not have a high false positive rate if the prevalence of the disease is low.
My understanding is that the PCR test, according the Nobel Laureate inventor of the process used in the test, makes a very good research tool, but a poor clinical diagnostic tool as it’s not a definitive, linear, positive or negative, and is not a “gold standard” test
Hence my question about the number of cycles. I’ve not heard one mention of this component of the process mentioned in the media, task force briefings, or even scholarly papers. All we hear is X quantitative numbers of “new cases” but never any mention of the qualitative aspects. How many cycles does it take to get a positive from a highly symptomatic patient? Is there any standard? When do they stop? Isn’t this THE data that’s steering the policy ship?
April 27th, 2020 at 12:21 AM ^
PCR is now the gold standard for much of diagnostic virology testing:
http://depts.washington.edu/uwviro/clinical-testing/
Although it is true that the test requires interpretation, that is true for much of laboratory medicine. The difference between well run labs and big box labs can be apparent in the interpretation of tests with gray areas. The number of cycles has to be validated for each lab because it depends on the equipment, reagents, etc. There is an example in the FDA guidance on page 36:
https://www.fda.gov/media/136472/download
Part of the frustration with the FDA and CDC handling of testing in February were the barriers imposed in getting these tests up and running at labs that know what they’re doing. Many of the questions that you raise are standard protocol for validation of new Laboratory Developed Tests. Experienced laboratory directors know how to get these tests validated quickly and effectively, but the FDA created significant, unnecessary barriers.
April 27th, 2020 at 12:24 AM ^
So, I went back and looked, and you were absolutely correct that I butchered the language; I work with statistics, but not diagnostics, so I'm better versed with the probability. :). I went back and altered it for future readers.
However, I'm pretty sure that I got the calculations of PPV correct. (I didn't actually calculate NPV). To walk through the first example again:
- Sample size, one million, with one hundred positive and the remainder negative
- Test is 99% sensitive -- out of 100 positive people, it will return 99 true positive results and 1 false negative result
- Test is 95% specific -- out of 999,900 negative people, it will return 49,995 false positive results and 949,905 true negative results
PPV is (true positives) / (true positives + false positives) = 99 / (99 + 49,995) = 0.2%
NPV is (true negatives) / (true negatives + false negatives) = 949,905 / (949,905 + 1) >> 99.9%
If you know the error rates for the test -- presumably computed by comparison to known samples or to the results of a more accurate test -- you can compute the population prevalence by working backward from your observations. It's just, the more accurate the test is (on both axes), the smaller the sample size you need to draw valid conclusions.
The problem is that people -- even doctors -- sometimes confuse the test specificity with the likelihood of any given positive test being correct. The test I've shown here -- which is a perfectly useful screening apparatus, if you know how to use it -- is a great example of that. If a doctor suspects this rare condition and issues the test, and the result comes back negative, it's time to come up with a new theory. But if the test comes back positive, you'll need another, likely more expensive or intricate test, to confirm it.
That’s so true about PPV, let’s hope that fact is remembered as more tests are available for the test, screen, trace strategy, which is likely in our future
This is a classic probability theory example. The variation I heard of was HIV testing - even though the test was very accurate, the thing they were looking for was so rare that false positives outweighed true positives by a lot. That has serious implications for diagnosis - if you get a positive result, what do you tell the patient, especially considering that at the time HIV infection was a near-fatal condition! - even though the test does a great job at estimating the infection rate within the population.
Like many other things, it's all about the context.
Isn’t this why duplicate and triplicate testing is performed?
April 27th, 2020 at 11:01 AM ^
Sort of? In this situation that I laid out, if you ran the same test twice, and you had one positive test and then one negative test, you'd conclude that the patient was negative because the likelihood of.a false negative was so low. However, if you had two positive tests, I don't think that changes the diagnosis any. The problem is, you can't differentiate between a true positive test and a systemic false positive.
Suppose that a test's false positive rate is due to the inability to distinguish between two similar biological agents, one of which is an indication of the rare syndrome that we've been discussing, and the other is innocuous. If the patient in question has this second agent, whatever it is, they're going to get a repeated false positive test; the problem is that the trials aren't really independent, so you can't make any new inferences from a second positive test. No new information has been gained.
Therefore, when you're testing for a rare condition, and you get a positive test, you need to run a different test -- preferably, something that uses an entirely different mechanism to detect the thing you're interested in.
I personally think the turn-around time on RT-PCR is overblown. Maybe that's just because I run so goddamn many PCR/qPCRs so they are second nature to me, but if you're just doing RT-PCR and electrophoresis, you should get results in like 4 hours at the latest.
But this Abbott thing intrigues me. I'm assuming the protocol is not publicly available, but is it effectively a RNA qPCR? And if so, how are they able to amplify the strand without cycling? Do they just add enough primer that you basically have just an elongation step and bank on there being a lot of template RNA?
April 26th, 2020 at 10:37 PM ^
Yeah hard to say about the Abbott test-- I don't think it's quantitative. It's slapping some primers on the RNA and cranking as many quick-and-dirty copies of the short-ish oligos you can get in a few minutes (I couldn't find anything that said how long the copy is) and you either get a signal or you get bubkus.
Hmmm, I'm not sure I like that. I always worry about sensitivity and noise when dealing with fluorescence, even if it's DNA specific like SYBR. I mean if it reliably works, it works, I just have questions about the specificity of the signal
The total time depends on the setting you are in. Getting a result in a research setting may be possible in 4 hours, but if it’s a quality (GLP) environment the results will get held up in quality review.
Great work man!
April 26th, 2020 at 10:52 PM ^
I was thinking of getting tested. cdc website says most people will have mild symptoms and testing won’t be necessary. Also there are testing sites for healthcare professionals but I’m not sure about my symptoms. I have diarrhea. Nothing else and my youngest son had a mild fever for half a day. Maybe tomorrow.
April 26th, 2020 at 10:53 PM ^
This is frickin awesome. I feel like I'm I'm a scientist just for reading it, or maybe by osmosis. Or something.
Not sarcasm, BTW, this is really cool, and impressive that one who is so versed in this can make it (somewhat) accessable to fools like me.
Phenomenal work, Sopwith! I’ve been writing about SARS-CoV-2 and immune response for the general public, and metaphor has been my constant companion.
Logged in to reiterate how outstanding these posts are.
Was just joking with a couple of my old med school buddies about how Immunology was one of the most difficult courses in all of med school because of the complexity. Now we have the general public trying to understand it with little to no background.
You have done a wonderful job of putting it in understandable and easy-to-read terms. Thank you
So one big consequence of the Stanford test is a lot of people are saying "See? this means the mortality rate is a lot lower than we thought." Is that actually true though, are the positives in the study all true positive cases that should count toward the denominator or mortality rate? In other words, can a person test positive in that study when not actually sick and simply have had some exposure?
Also, thank you for doing these, they're literally the best thing I've read on the internet on the subject... and I've looked.
April 27th, 2020 at 11:10 AM ^
The Stanford study in particular had potential issues from the self-selection of participants.
That said, you're splitting hairs. If a person "had some exposure" but didn't get "sick" -- i.e., the virus didn't take hold -- does that count as an infected patient? The answer probably depends upon what answer you want the data to support.
My answer would be yes, and doubly so if it turns out that exposure yields immunity, something we're all hoping. That patient was exposed, developed antibodies, and fought off the infection -- they should be counted in the statistics as an asymptomatic case.
April 27th, 2020 at 10:09 AM ^
Great stuff OP. My wife is getting an antibody test this week from her doctor. We were all sick as fuck, twice, in Feb and March, and it's entirely possible that we had it given where we live.
hatter, if you don't mind, can you please let us know how the test turns out?
Absolutely.
